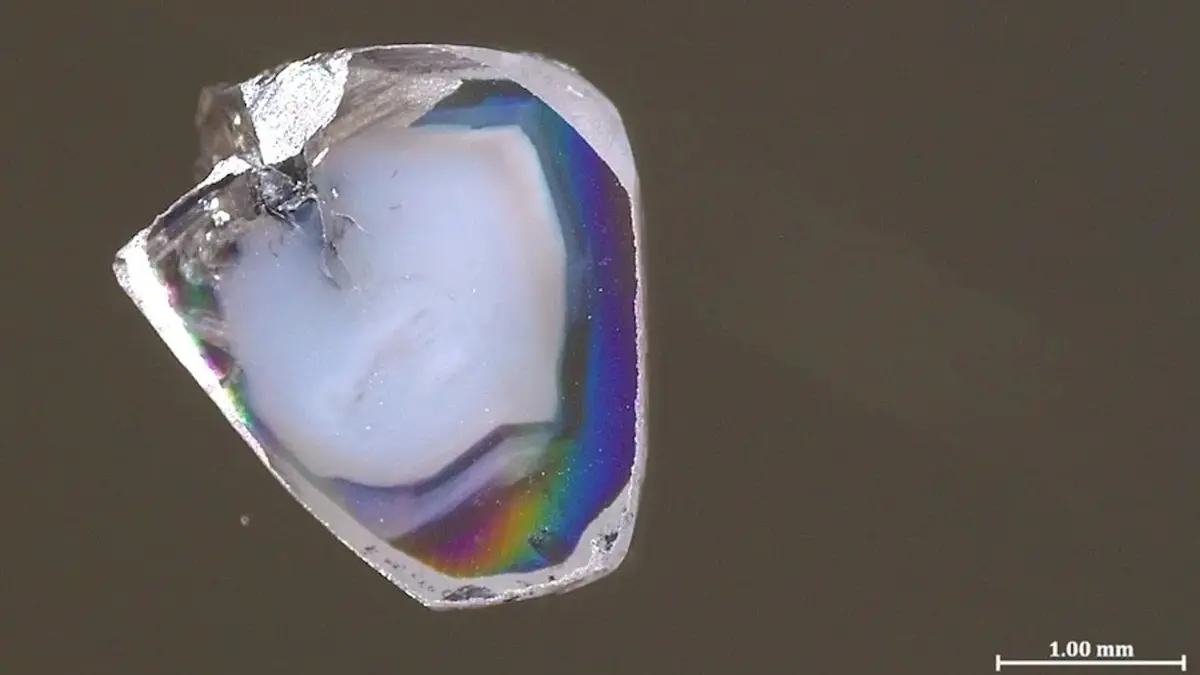
 In a mine in South Africa, miners have unearthed a pair of diamonds containing controversial chemical compounds. This discovery is expected to shed new light on how are formed.
In a mine in South Africa, miners have unearthed a pair of diamonds containing controversial chemical compounds. This discovery is expected to shed new light on how are formed.
These two samples of the precious mineral, formed hundreds of kilometers deep in the Earth’s plastic , contain inclusions of materials that form under completely opposite chemical conditions. Researchers have described this unusual combination as “practically impossible.”
What Did Scientists Discover?
Like many other gemstones, these two diamonds contain so-called inclusions—tiny particles of surrounding rock trapped during the diamond formation process. Most dislike such inclusions, but for scientists, they are an intriguing source of information. This is especially true for diamonds formed very deep—within the inaccessible mantle—because they bring these inclusions to the surface almost untouched. In other words, the minerals rise hundreds of kilometers upward, virtually unchanged, as noted by Live Science.
Each of the two discovered samples contains inclusions of carbonate minerals rich in oxygen atoms (a state known as oxidized) as well as oxygen-poor nickel alloys (a state known as reduced). Just as an acid and a base react instantly to form water and salt, oxidized carbonate minerals and reduced metals do not coexist for long. Typically, inclusions in diamonds contain only one of these components. So, it’s no surprise that their simultaneous presence puzzled Yakov Weiss, a senior lecturer in Earth Sciences at the Hebrew University of Jerusalem and the lead author of the study.
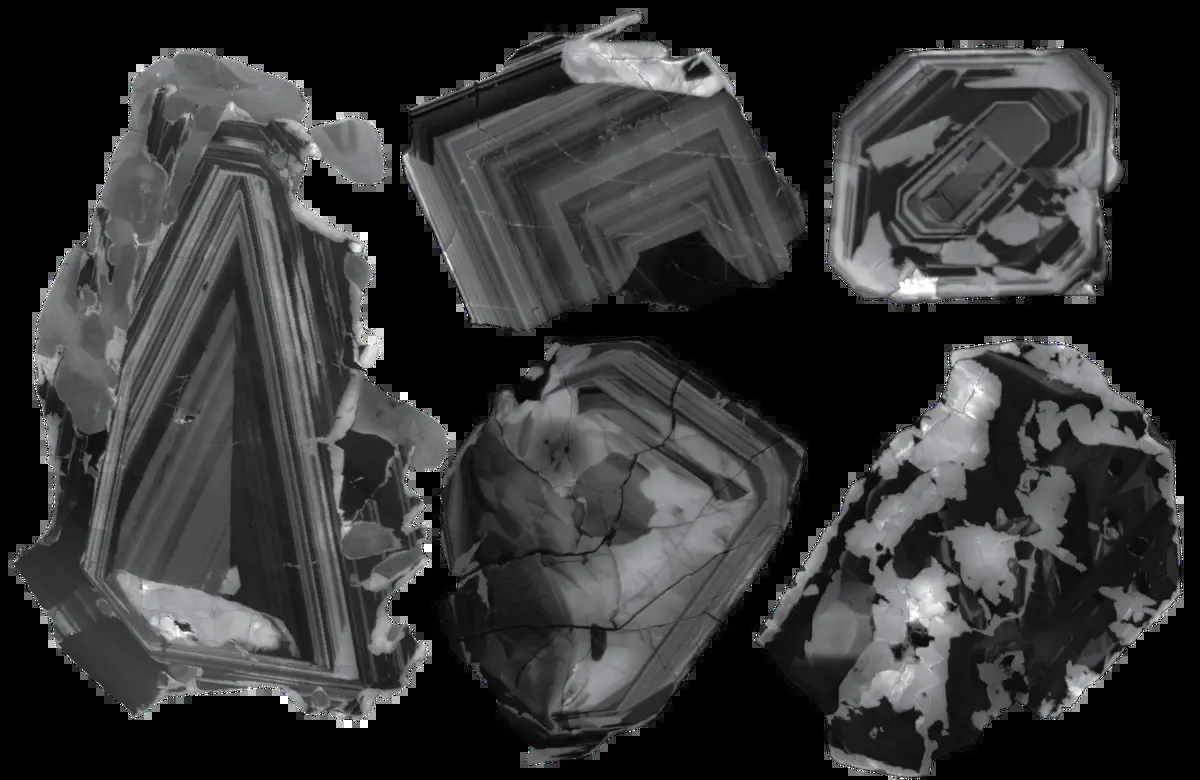
Weiss and his team were so baffled that they even postponed studying the findings for a while. Upon later reanalyzing the diamonds, the researchers discovered that the inclusions reflect a snapshot of the reaction that led to the formation of these sparkling stones. Thus, scientists have confirmed for the first time the possibility of diamond formation through the interaction of carbonate minerals and reduced metals in the mantle.
Thanks to the new samples, scientists have been able to observe the middle of this reaction in a natural diamond for the first time. “Essentially, these are two sides of the oxidation spectrum,” Weiss noted in the report. This discovery is significant for understanding what happens in the mysterious center of the mantle. As you go deeper into the Earth, moving away from the surface, rocks and minerals become more reduced, and the amount of oxygen molecules decreases. However, direct evidence of this change in the mantle is virtually nonexistent.
The two new diamonds, originating from depths of 280 to 470 kilometers below the Earth’s surface, represent the first verification of theoretical data regarding mantle chemistry. According to Weiss, one conclusion is that oxidized molten material lies deeper than previously thought. Kimberlites—the rocks that erupt alongside diamonds—are oxidized. Therefore, researchers believed they could not form at depths significantly lower than 300 kilometers. However, the results obtained have led scientists to question this assumption.
Weiss stated that diamond formation reactions likely occur when carbonate liquids are pulled down by tectonic plates, causing contact between minerals with high oxygen content and metal alloys in the mantle.
The study’s results were published in the journal Nature Geoscience.
